Abstract
Background
Absolute lymphocyte count (ALC)is known to be an independent prognostic factor for overall survival (OS) in patients receiving autologous transplantation for Hodgkin's and non-Hodgkin's lymphomas, multiple myeloma and breast cancer. In acute myeloid leukemia (AML) patients, enhanced ALC recovery after intensive chemotherapy (IC) has been associated with superior OS and leukemia-free survival (LFS). However, few if any data correlated ALC recovery with novel therapies as well as with updated prognostic factors, such as European Leukemia Net (ELN) risk-classification.
Aims
Our study aims at evaluating the predictive value of ALC recovery in newly diagnosed AML patients who obtained complete remission (CR) to IC by analysing its impact through different patient subgroups according to therapy response, type of chemotherapy regimen and the use of allogeneic stem cell transplantation (HSCT).
Methods
We evaluated 156 responsive AML patients treated with IC at Bologna Seràgnoli Hematology Institute since 2007. We defined 4 ALC time-points (TPs): at 15, 21 and 28 days from the start of induction chemotherapy and before consolidation therapy (CC). ALC cut-off was established at 500/mm3. In addition to the assessment of the single TP, patients were also grouped in those who obtained ≥500/mm3ALC in all 4 TPs and those who had<500/mm3ALC in at least one TP. Median follow-up was 65 months.
Results
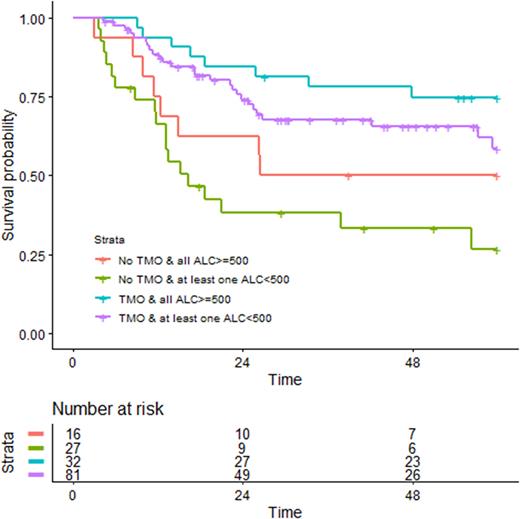
When ALC recovery was assessed in whole population, both at single TPs and ALC recovery achievement at all TPS vs others, no statistically significant association with better OS and LFS has been shown. Then we subdivided patients according to IC regimen administered: "3+7-based" regimen (52,6%) versus those who were treated with "fludarabine-based" regimen (47,4%). Considering "3+7" patients, at TP day 15 a significant correlation with OS (p=0.047) of patients with ≥500/mm3ALC (34,6%) vs those who had<500/mm3ALC (17,9%) was observed (instead for ALC-21 p value was 0.078). A similar trend (p=0.021) was shown for those who obtained ≥500/mm3 ALC in all 4 TPs (27,5%) compared to those who had <500/mm3ALC in at least one TP (25%). Of note, FLT3-ITD positive patients in this subgroup, who achieved early ALC recovery at day 15 and at all 4 TPs assessment, had better outcomes, despite with p=0.27 and p=0.19 respectively, because of the meager number of patients. Among patients treated with "fludarabine-based" regimens, ALC recovery assessment did not show remarkable correlation with outcome, even when considering the NPM1 mutated subgroup. We, then, analysed in whole population the impact of post-chemotherapy ALC recovery in patients who received HSCT (63,5%) vs those who did not (36,5%) (Fig.1). As expected, transplanted patients had globally better outcome than patients who received only chemotherapy. In patients who received HSCT the impact of ALC recovery after chemotherapy was minimal (blue and purple curve). In contrast, patients who had ALC <500/mm3 ALC in at least one TP and did not undergo HSCT had the worst outcome (green curve). Interestingly, patients who were not transplanted but had ≥500/mm3ALC in all 4 TPs (red curve) had a better OS, comparable to transplanted patients.
Conclusions
Our study indicates ALC recovery after IC as a promising survival predictor in AML patients, especially in those who do not undergo HSCT. It is reasonable to underline the impact of fludarabine, as for its lymphocytolytic effect, in order to explain the different results in the subgroup fludarabine-based. The outcome trends in different prognostic subgroups according to ELN classification, such as FLT3, NPM1, adverse cytogenetics, need a caseload expansion. In the path that aims to investigate the complex interaction between leukemia and the immune system, our study may provide the clinical rationale for the construction of an immunological score to be integrated in the current disease-centered risk classification system. Future studies in larger cohorts addressing the impact of ALC recovery in association with the use of novel agents and minimal residual disease status are highly warranted.
Disclosures
Papayannidis:Abbvie: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Blueprint: Honoraria; Incyte: Honoraria; GlaxoSmithKline: Honoraria; Bristol Myers Squibb: Honoraria. Cavo:AbbVie, Amgen, Bristol Myers Squibb/Celgene, Pfizer, GlaxoSmithKline, Sanofi, Roche, Takeda: Consultancy, Honoraria; Janssen: Honoraria, Speakers Bureau. Curti:Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal